RESEARCH ARTICLE |
https://doi.org/10.5005/jp-journals-10016-1294 |
Stage-based FGR (Barcelona Protocol): Perinatal Outcome in SGA and FGR
1,2Department of Fetal Medicine, Fernandez Hospital, Hyderabad, Telangana, India
Corresponding Author: Suseela Vavilala, Department of Fetal Medicine, Fernandez Hospital, Hyderabad, Telangana, India, Phone: +91 9866611856, e-mail: drsuseela@gmail.com
Received on: 11 May 2022; Accepted on: 04 July 2022; Published on: 28 December 2022
ABSTRACT
Objective of the study: To identify adverse perinatal outcomes, these include stillbirth, neonatal death, hypoxic-ischemic encephalopathy, need for mechanical ventilation, or severe metabolic acidosis in small for gestational age (SGA) and in all stages of fetal growth restriction (FGR) based on Barcelona Protocol.
To evaluate the demographic variables, maternal risk factors, mode of delivery, birthweight, and indications of operative delivery in SGA and all stages of FGR.
Materials and methods: It is a prospective observational study underwent from January 2019 to June 2019 at the Department of Fetal Medicine, Fernandez Hospital. All mothers with singleton pregnancies, who came for fetal growth scan, it’s mandatory to have expected delivery date (EDD) confirmed in first trimester itself and estimated fetal weight (EFW) < 10th percentile were included in the study.
Multiple pregnancies, structurally abnormal fetuses, first scan >20 weeks (GA not accurate) were excluded from the study.
Mothers with EFW < 10th percentile underwent serial sonographic evaluation of estimated fetal weight at 2-weekly intervals including multivessel Doppler assessment based on staged-based protocol. If the EFW 3–10th percentile, multivessel Doppler findings are within normal range the fetus is termed as SGA fetus and is followed up every 2 weeks. If the EFW < 3rd percentile or any of the multivessel Doppler findings show features of placental insufficiency, the fetus is termed as FGR, and management is based on the stage-based Barcelona Protocol. Maternal, fetal, and neonatal characteristics, neonatal morbidity, and adverse perinatal outcome were recorded.
Results: Among 6,240 mothers who underwent growth scans during the study period, 14% (n = 858) with EFW < 10th percentile were taken as the study population. A total of 768 pregnant women were included in the study. Based on Barcelona protocol, 68% (n = 521) and 32% (n = 247) were termed FGR and SGA, respectively. FGR fetuses were classified into four stages —488 (95%), 23(14.4%), 10(2%), I, II, III, and IV, respectively.
Among 247 pregnant women with SGA fetuses, 42% required induction of labor, FGR stage I (488), 40% required induction of labor. FGR stage II & III fetuses (25) 27% required induction of labor.
Fetuses grouped under stages II & III have 61% admissions into NICU, compared to 12% in FGR stage I and 2% SGA group fetuses.
There are no adverse perinatal outcomes in SGA group.
In FGR stage I group, adverse perinatal outcomes in terms of metabolic acidosis, 5 minutes APGAR < 7, hypoxic ischemic encephalopathy (HIE), need for mechanical ventilation are 1.8%, 0.6%, 0.6%, 0.8%, respectively.
In FGR stage II & III, metabolic acidosis was diagnosed in five (15%) neonates, two neonates (6%) required mechanical ventilation with four (12%) of stillbirths. There is one neonatal death (NND) in the entire cohort, classified under FGR stage I.
Conclusion: Incorporation of Barcelona protocol as a structured antenatal surveillance protocol discriminates between SGA fetuses and stages of FGR. Prenatal recognition of FGR allows for close monitoring and timely delivery. There is a higher risk for adverse perinatal outcomes in FGR II & III compared to FGR stage I and SGA fetuses.
How to cite this article: Kachakayala M, Vavilala S. Stage-based FGR (Barcelona Protocol): Perinatal Outcome in SGA and FGR. Int J Infertil Fetal Med 2022;13(3):101-110.
Source of support: Nil
Conflict of interest: None
Keywords: Fetal growth, Fetal growth restriction (FGR), Multivessel fetal Doppler, Perinatal outcome, Small for gestational age (SGA).
INTRODUCTION
Small fetuses are defined as those with an ultrasound estimated weight below a threshold, most commonly the 10th percentile which includes both SGA and FGR fetuses.1 SGA fetuses are defined as those with an estimated fetal weight less than the 10th percentile for gestational age.2-4 FGR is a failure of the fetus to reach its full growth potential and is associated with maternal, placental, and fetal conditions, including hypertension, and other placental deficiencies.5 Suboptimal intrauterine growth affects up to 10% of pregnancies and confers an increased risk of perinatal morbidity and mortality.6 It increases the risks of stillbirth, birth hypoxia, neonatal death, and neurodevelopmental impairment.7
The current birth rate for India is 17.8 births per 1,000 people.8 The current perinatal mortality rate of India is 26 per 1,000 births.9 It ranges from 16 per 1,000 births in urban areas to 28 per 1,000 births in rural areas. The stillbirth rate in India is 22 per 1,000 births and varies from 20 to 66 per 1,000 births in different states. The commonest cause of stillbirths is FGR (27.9%).10
Current antenatal detection rates of FGR are reported at 20–25%.11,12 Screening for FGR and identification with timely intervention has been an accepted strategy for reducing the stillbirth rate.13 Therefore, a preventive strategy to reduce stillbirths is to improve the antenatal detection of fetal growth failure.6 Whenever FGR is diagnosed prenatally, increased surveillance and timely delivery aim to improve perinatal outcome in FGR, balancing the risk of antepartum stillbirth by remaining in utero and iatrogenic prematurity which potentially causes significant morbidity or neonatal death by too early intervention.
Fetal growth is monitored using ultrasound to differentiate between constitutionally small, but normal fetuses and those with restricted growth.5,14 A systematic approach to FGR, which entails a proper identification of FGR vs SGA, and a stage-based management protocol that may help in reducing clinical variability is much needed.1
Hence a protocol that distinguishes FGR from SGA, will ascertain whether there is a risk of in utero fetal injury or death and integrates the best available evidence which helps in reducing clinical practice variation was proposed by Gratacos and Figueras—stage-based management protocol, Barcelona. This was incorporated into our institution’s clinical practice in January 2018.
The current study aims to identify perinatal outcomes in terms of stillbirth and neonatal death in the study population and to evaluate the demographic variables, maternal risk factors, mode of delivery, birth weight, and indications of operative delivery in SGA and all stages of FGR. Neonatal morbidity was assessed in terms of a 5 minutes APGAR score of less than 7, delivery with metabolic acidosis, or admission to the neonatal intensive care unit.
MATERIALS AND METHODS
It is a prospective observational study that underwent from January 2019 to June 2019 at the Department of Fetal Medicine, Fernandez Hospital, a tertiary perinatal referral center. The study was approved by the Institutional Review Board and Ethical Committee of Fernandez Hospital.
Mothers with singleton pregnancies with EFW < 10th percentile, gestational age > 26 weeks and EFW > 650 G were included in the study. Gestational age confirmation is done by CRL of the fetus. Mothers with EFW < 10th percentile were categorized into SGA and FGR, depending on multivessel Doppler. If the EFW is between 3rd and 10th percentile, and multivessel Doppler findings are within normal range, then the fetus is followed up every two weeks and is termed as SGA fetus.
If the EFW < 3rd percentile or any of the multivessel Doppler findings show features of placental insufficiency, then the fetus is termed as FGR, and management is based on the stage-based Barcelona protocol.
If the EFW < 3rd percentile or the cerebroplacental ratio < 5th percentile or uterine artery PI > 95th percentile or middle cerebral artery PI < 5th percentile, then the fetus is classified under stage I FGR, where the fetus is followed up weekly along with maternal evaluation of hypertension and delivered by 37 weeks of gestation.
If the umbilical artery shows absent end-diastolic flow (AEDF) or reversal in aortic isthmus Doppler then the fetus is termed as stage II FGR and fetus is followed up in 2–3 days and delivered by 34 weeks of gestation by cesarean section after administration of antenatal steroids (Inj. Betamethasone 12 mg 24 hours apart intramuscular) if GA of delivery < 34 weeks.
If the umbilical artery shows reversed end diastolic flow (REDF) or ductus venosus PI > 95th percentile then the fetus is termed as stage III FGR and the fetus is followed up daily and delivered by 30 weeks of gestation by cesarean section after administration of antenatal steroids (Inj. Betamethasone 12 mg 24 hours, apart intramuscular) if GA of delivery < 34 weeks and MgSO4 for neuroprotection if GA of delivery < 32 weeks.
If ductus venosus Doppler shows a wave reversal, then the fetus is termed as stage IV FGR and fetus is followed up to every 12th hour, and delivery is done by 26 weeks
The Doppler findings were checked 12h/24 hr intervals before decision-making (Table 1 and Fig. 1).
| Stage | Path physiological correlate | Criteria (any of) | Monitoring | GA/mode of delivery |
|---|---|---|---|---|
| I | Severe smallness/mild placental insufficiency | EFW < 3rd percentile, CPR < p5, UtPI>p95,UmPI>p95,M CAPI>p95 | Weekly | 37 weeks Labor induction |
| II | Severe placental insufficiency | UAAEDF reverse AoI | Biweekly | 34 weeks Cesarean section (CS) |
| III | Low suspicion fetal acidosis | UAREDF DV-PI > p95 | 1–2 days | 30 weeks CS |
| IV | High suspicion fetal acidosis | DV reverse a flow, cCTG < 3 ms. FHR decelerations | 12 h | 26 weeks CS |
CPR, cerebroplacental ratio; DV, ductus venosus; FHR, fetal heart rate UAAEDF, umbilical artery absent end-diastolic flow; UAREDF, umbilical artery reversed end-diastolic flow

Figs 1A to E: FHR, placenta position, BPD, HC, AC, FL planes
Multiple pregnancies, fetuses with chromosomal abnormalities, structurally abnormal fetuses, first scan >20 weeks (GA not accurate), early onset FGR (<26 weeks of gestation/EFW <650 G) were excluded from the study.
The following variables were assessed at each scan—fetal heart rate, presentation and lie, amniotic fluid index (AFI), placenta, fetal biometry HC/AC/FL, estimated fetal weight, and multivessel Doppler which includes assessment of uterine artery PI,15 umbilical artery PI,15 middle cerebral artery PI, C/P ratio, aortic isthmus waveform,16 ductus venosus PI15 (Figs 2 and 3 and Flowchart 1). All variables were measured as per the International Society of Ultrasound in Obstetrics & Gynecology (ISUOG) guidelines.17

Figs 2A to E: Normal uterine artery Doppler, normal umbilical artery Doppler, normal MCA Doppler, normal aortic isthmus Doppler, normal ductus venous Doppler
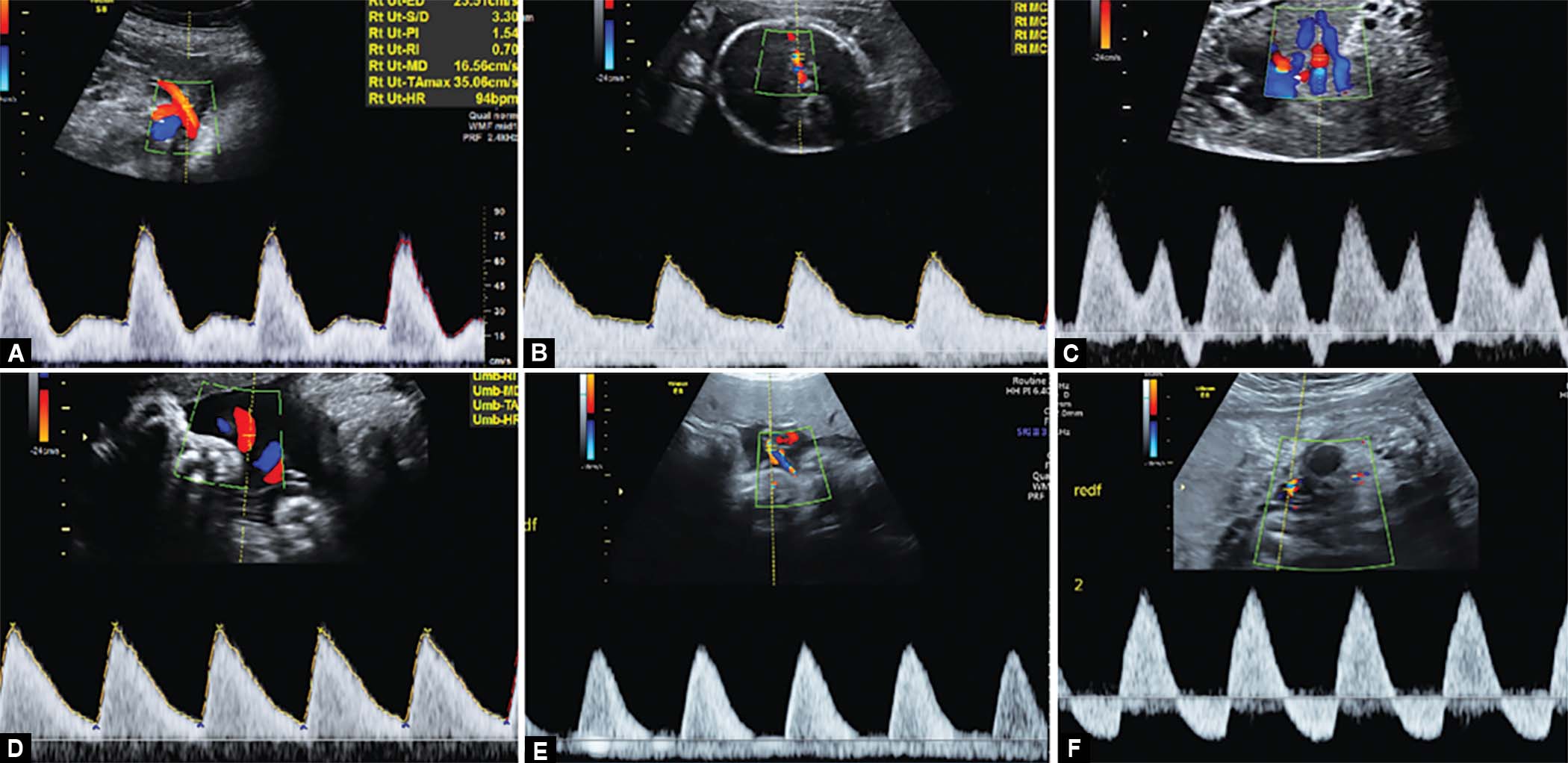
Figs 3A to F: Uterine artery Doppler with increased resistance, redistribution in MCA Doppler, ductus venosus Doppler with ‘a’ wave reversal, umbilical artery Doppler with increased resistance, AEDF in umbilical artery, REDF in umbilical artery

Flowchart 1: Stage-based management protocol for FGR fetuses17
Ultrasound examinations are performed with Voluson E8/E6/S8 expert of GE Company with curved array transabdominal transducer 3.5–5.0MHz. All ultrasound examinations are performed by fetal medicine consultants/trained obstetricians who are accredited by the Fetal Medicine Foundation, UK.
All cases were prospectively recruited. Astraia database (fetal medicine) and electronic medical records were reviewed for follow-up. Maternal, fetal, and neonatal characteristics included maternal age, parity, body mass index. medical and obstetrical risk factors, EFW before delivery, multivessel Doppler findings, gestational age of diagnosis and delivery (in weeks), mode of delivery, indication for induction of labor and lower segment cesarean section (LSCS), cord arterial blood gas (ABG), birth weight, the APGAR score, need for resuscitation, NICU stay, neonatal morbidity and adverse perinatal outcome were recorded.
EFW is calculated using Hadlocks Formula (BPD, HC, AC & FL) in Astraia Software.
The following population growth charts were incorporated in Astraia software. Snijders et al. Ultrasound Obstet Gynaecol 1994; 4:34–48 were used for BPD, HC, AC, FL and Yudkin et al. Early Human Dev 1987; 15:45–52 were used for EFW. These charts are not validated for Indian population.
We defined neonatal morbidity as one or more of the following criteria: a 5 minutes APGAR score of less than 7, delivery with metabolic acidosis (defined as a cord blood pH < 7.1 and base deficit > 10 mmol/L), or admission to the neonatal unit (defined as admission < 48 hours after birth and discharge ≥ 48 hours after admission).
Adverse perinatal outcome is defined as stillbirth or term livebirth associated with neonatal death, hypoxic-ischemic encephalopathy, need for mechanical ventilation, or severe metabolic acidosis (defined as a cord blood pH < 7.0 and base deficit > 12 mmol/L: the criteria by which international guidelines define fetal metabolic acidosis, which can be regarded as cause for cerebral palsy during childhood).18
Descriptive analysis was done for SGA and all stages of FGR during the study period. Evaluation of perinatal outcomes is done by statistical proportion and Chi-square tests. The significance level for all tests was p < 0.05.
RESULTS
Sample Size
Among 6,240 mothers who underwent growth scans during the study period (Jan 2019–June 2019), 14% (n = 858) with EFW < 10th centile taken as the study population. A total of 28 were excluded as chromosomal or structural abnormalities were present; 62 were not included in the study as they were not delivered at our institution leaving 768. Based on Barcelona protocol definition for FGR and SGA, 68% (n = 521) and 32%(n = 247) were termed FGR and SGA, respectively. FGR fetuses were classified, depending on stage-based classification and based on the last scan (EFW before delivery, multivessel Doppler) findings into four stages—stage I 488 (95%), stage II 18(3.5%), stage III 7(1.5%), and stage IV 0 fetuses (Flowchart 2).

Flowchart 2: Description of study population
Interpretation
The maternal characteristics (demography and risk factors) for each group are displayed in Table 2.
| SGA (N = 247) | FGR I (N = 488) | FGR II–III (N = 33) | |
|---|---|---|---|
| Mean maternal age | 27 | 28 | 27 |
| Primigravida | 135 (55%) | 263 (54%) | 15 (45%) |
| Multigravida | 112 (45%) | 225 (46%) | 18 (55%) |
| Mean BMI (Kg/m2) | 25 | 26 | 26 |
| Mean gestational age of diagnosis (weeks) | 35 | 33 | 30 |
| Mean GA at delivery (weeks) | 38 | 36.5 | 31 |
| Hypertensive disorders | 19 (8%) | 100 (20%) | 7 (21%) |
| PGDM/GDM | 19 (8%) | 72 (15%) | 2 (6%) |
| Hypothyroidism | 13 (5%) | 39 (8%) | 5 (15%) |
| Other medical disorders | 20 (8%) | 59 (12%) | 1 (3%) |
Advanced maternal age (>35 years) is similar in both groups 5% (12) in pregnant women SGA and 3% (16) in pregnant women with FGR. High BMI (>30) are 18% (44) in pregnant women with SGA and 14% (71) in pregnant women with FGR.
Pregnant women with SGA and stage I FGR fetuses are more likely nulliparous when compared to pregnant women with stages II & III fetuses who are more likely to be multiparous.
Maternal comorbid conditions like hypertensive disorders of pregnancy, PGDM, GDM, hypothyroidism and other medical risk factors are more in pregnant women with FGR (45% in FGR II - 55% in FGR 1 & 45% in FGR II & III fetuses) compared to 29% in pregnant women with SGA fetuses. hypertensive disorders of pregnancy (gestational hypertension, pre-eclampsia, chronic hypertension, and eclampsia) is the most common medical risk factor in all the groups.
The perinatal characteristics for each group are displayed in Table 3.
| SGA (N = 247) | FGR I (N = 488) | FGR II–III (N = 33) | |
|---|---|---|---|
| Mean EFW before delivery (gm) | 2358 | 2043 | 1054 |
| Mean birth weight (gm) | 2400 | 2154 | 1073 |
| Mean APGAR at 5 min | 9 | 9 | 8 |
| Mean arterial pH | 7.27 | 7.24 | 7.20 |
| No. of NICU admissions | 5 (2%) | 57 (12%) | 20 (61%) |
The mean estimated fetal weight and mean neonatal birth weight after delivery are comparable in all groups (2358/2400; 2043/2154; 1054/1073). Perinatal characteristics like mean APGAR at 5 minutes (9), mean arterial pH (7.2) are similar in all groups.
Fetuses grouped under stages II & III have 61% admissions into NICU, compared to 12% in FGR stage 1. This correlates with the severity of FGR.
About 61% admissions into NICU in stages II & III, this insight would help in future prognostication and couple counseling.
Obstetric outcomes and neonatal outcomes for each group are displayed in Tables 4 and 5.
| SGA (N = 247) | X2 | p-value | FGR1 (N = 488) | X2 | p-value | FGR II–lll (N = 33) | p-value | |
|---|---|---|---|---|---|---|---|---|
| Spontaneous labor <37 weeks |
14 (6%) | 8.48 | 0.003 | 4 (0.8%) | 7.35 | 0.06 | 0 | * |
| Spontaneous labor >37 weeks |
33 (13%) | 16 (3%) | 9 (27%) | |||||
| IOL < 37 weeks | 48 (19%) | 0.77 | 0.37 | 181 (37%) | 122.4 | <0.001 | 0 | * |
| IOL > 37 weeks | 56 (23%) | 37 (7.2%) | 0 | |||||
| Elective LSCS<37 weeks |
25 (10%) | 1.55 | 0.21 | 67 (14%) | 0.92 | 0.33 | 17 (57%) | * |
| Elective LSCS >37 weeks |
34 (14%) | 57 (12%) | 0 | |||||
| Emergency LSCS<37 weeks |
19 (8%) | 0.02 | 0.86 | 67 (14%) | 0.58 | 0.44 | 7 (21%) | * |
| Emergency LSCS>37 weeks |
1 8 (7%) | 59 (12%) | 0 |
*p-value could not be calculated in Chi square tests when one of the variable is 0, hence did not mention and replaced by asterisks in the table.
| Neonatal morbidity | Adverse perinatal outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NICU stay (Days) | Metabolic acidosis | p-value | 5 min APGAR <7 | p-value | HIE | p-value | Need for mechanical ventilation | p-value | neonatal death | p-value | Still-birth | p-value | Perinatal deaths | |
| SGA (N = 247) | 4 | 0 | * | 0 | * | 0 | * | 1 | <0.001 | 0 | * | 0 | * | 0 |
| FGR I (N = 488) | 7.6 | 9 (1.8%) | <0.001 | 3 (0.6%) | <0.001 | 3 (0.6%) | <0.001 | 4 (0.8%) | <0.001 | 1 | <0.001 | 0 | * | 0.2% |
| FGR II & III (N = 33) | 19 | 5 (15%) | 0.001 | 0 | * | 0 | * | 2 (6%) | 0.004 | 0 | * | 4 | 0.005 | 12% |
*p-value could not be calculated in Chi square tests when one of the variable is 0, hence did not mention and replaced by asterisks in the table.
Among 247 pregnant women with isolated SGA fetuses, 29% delivered spontaneously, 42% required induction of labor, and 39% underwent cesarean section.
A statistically significant difference is observed in before and after 37 weeks in SGA fetuses. However, there is no significant difference is observed in Induction of labor, elective LSCS, or emergency LSCS before and after 37 weeks in this group.
In pregnant women with FGR stage I (488), 52% of women underwent cesarean section, 40% required induction of labor, and 8% of women delivered spontaneously. The most indication for cesarean section in this group is presumed fetal compromise, this explains the high incidence of cesarean section in stage I FGR.
A statistically significant difference is observed in spontaneous delivery and in induction of labor before and after 37 weeks in FGR fetuses. However, no significant difference is observed in elective LSCS, or emergency LSCS before and after 37 weeks in this group.
Around 73% of pregnant women with FGR stages II and III fetuses underwent cesarean section, rest 27% required induction of labor. A statistically significant association could not be determined in this group.
The most common indication for cesarean section is presumed fetal compromise in pregnant women with SGA (27%) & FGR stage I (56%) group, whereas Doppler compromise is the common indication in pregnant women with FGR stages II & III. The other common indications in all the groups were previous LSCS (25%; 10%; 8%), maternal request (18%; 9%; 8%) and maternal indication (8%; 7%; 21%) (Fig. 4).

Fig. 4: Indication for cesarean section in the study population
NICU admissions were 2% in the SGA group with a mean NICU stay of 4 days. There are no other adverse perinatal outcomes in this group.
In the FGR stage I group, 12% (57) NICU admissions with a mean NICU stay of 7 days. Adverse perinatal outcomes in terms of metabolic acidosis in 1.8%, 5 minutes APGAR < 7 in 0.6%, HIE in 0.6%, need for mechanical ventilation in 0.8%.
High rates of NICU admission (61%) in FGR II & III group with a mean NICU stay of 19 days. Metabolic acidosis was diagnosed in 15% (5) neonates, 6% (2) neonates who required mechanical ventilation with 12% (4) of stillbirths in this group.
A statistically significant association was found for the need for mechanical ventilation (p < 0.001) in SGA group.
A statistically significant association was found for metabolic acidosis (p < 0.001), 5 minutes APGAR (p < 0.001), HIE (p < 0.001), need for mechanical ventilation (p < 0.001) and neonatal death (p < 0.001) in stage I FGR.
A statistically significant association was found for metabolic acidosis (p < 0.001) need for mechanical ventilation (p < 0.001) and still birth (p < 0.001) in stages II & III FGR.
The commonest indication for NICU admission in all groups was respiratory distress syndrome. The other common indications in FGR groups were SS/NEC/pneumonia, retinopathy of prematurity (ROP), disseminated intravascular coagulation (DIC), transient tachypnea of newborn (TTNB) apart from prematurity (Fig. 5).

Fig. 5: Indications for NICU admissions in study population
There is one NND in the entire cohort, classified under FGR stage I antenatally with no maternal risk factors, increased resistance in umbilical artery Doppler, anhydramnios [secondary to chronic preterm premature rupture of membranes (PPROM)], delivered at 31 weeks of gestation by elective LSCS in view of previous LSCS, birth weight of 900 gms, 5 minutes APGAR of 8, cord pH 7.2, succumbed at 21 days of life in view of fetal inflammatory response syndrome.
DISCUSSION
The prevalence of FGR and SGA in our institution during the study period was 9.1% and 4.5%, respectively.
Advanced maternal age did not appear to be a risk factor in comparison with Nihal et al.19
It is generally accepted that nulliparity increases the risk of SGA infants when compared with multiparity,19-21 In this study, no association was observed between the number of previous deliveries and an increase in the frequency of SGA & FGR fetuses in comparison with Teixeira et al.22
In the SGA group, the rate of hypertensive disorders (8%) was higher compared to other medical disorders in comparison with Nihal et al. Hypertensive disorders of pregnancy (20%; 21%), thyroid dysfunction (15%; 6%), and gestational diabetes (15%:6%) were noted in pregnant women with stages I, II, & III FGR fetuses, respectively like Satyavrathan V et al. and Sharma et al.23,24
The mean gestational age of diagnosis for SGA and stages of FGR I, II, & III in the study was 35, 33, 30 weeks, respectively. However, the mean gestational age of diagnosis for FGR in Heera et al. was 34–35 weeks, Malhorta et al. was 27 weeks for FGR (Doppler abnormal) and 37 weeks for SGA group (Doppler normal).25
The mean GA at delivery in SGA was 38 weeks, in FGR stage I group was 36 weeks compared to 31 weeks in FGR stages II & III, whereas in Heera et al. study, the mean GA of delivery was 37(Doppler normal) and 27 weeks (Doppler abnormal), respectively.26 Preterm birth rate is more in FGR group in view of growth restriction and Doppler compromise.
LSCS rates in pregnant women with SGA fetuses were 62%, FGR stage 1 was 51% and 72% in stages II & III like Seal A et al. with 68% LSCS, Heena et al. with 75% LSCS rates. 24% of women delivered vaginally compared to 32% as in Seal A et al.29 Nearly 39% of pregnant women with SGA fetuses, 49% of pregnant women with FGR fetuses and 28% with FGR stages II & III fetuses, could deliver vaginally in our study compared to 32% in Seal A et al. and 24% in Heera et al. study.
The most common indication for LSCS in our study was presumed fetal compromise in SGA (26%) and FGR stage I group (45%) and in FGR stages II & III it is Doppler compromise (50%), whereas abnormal Doppler findings are the commonest indication observed in Raja Rajeswari et al., Heera et al., and Sinha et al.30
The preterm birth rate in our study was around 35% in SGA and FGR stage I group compared to 100% in FGR stages II & III group, this result is also statistically significant.
This high preterm birth rate in stages II & III is expected as there is an initiation of birth if the fetal condition worsens to prevent the risk of hypoxia and major morbidities, this is also correlating with NICU admissions which were 2% and 12% in SGA and FGR stage I group compared to 61% in FGR stages II & III group.
The mean birth weight was 2400 gm in SGA, in FGR stage I is 2154 gm in and was similar to Heera et al.25 The mean birth weight in FGR stages II & III is 1073 gm in comparison with Malhotra et al. and Sinha et al. So, SGA and stage I FGR had better birthweights than FGR stages II & III.25
Mean APGAR at 5 minutes was more than 7 in all groups similar to Heera et al.
Common indications for NICU admission in all three groups was RDS and prematurity alone similar to Fratelli et al.27
Adverse perinatal outcomes in the study (stillbirth or term livebirth associated with neonatal death, hypoxic-ischemic encephalopathy, need for mechanical ventilation, or severe metabolic acidosis) is 4.35% which is similar to Dwyer et al. The commonest adverse outcome is stillbirth in FGR (II & III) group, need for mechanical ventilation in FGR I group compared to neonatal sepsis in both groups observed in study done by Dwyer et al.
The commonest cause neonatal morbidity in the study is metabolic acidosis (1.8%,15%) in FGR (I, II, & III) groups, whereas there is no specific cause for neonatal morbidity in SGA group.
Adverse perinatal outcome of 0.4% in pregnant women with SGA group, 4.4% in pregnant women with stage I FGR fetuses, 33% in FGR stages II & III fetuses compared to 1.3% in FGR with normal Doppler group, 11.5% in FGR with abnormal Doppler in Dwyer et al. study.28
Perinatal deaths in the study are 0.65% (0; 0.2%; 12%) compared to 0.54% in PORTO study. The survival rate in the study corresponds to 99.35% (100%; 99.7; 88%) compared to 96.5% in the study done by Arpita et al.11
There is a statistically significant association for adverse perinatal outcomes in SGA group and stage I FGR group which emphasizes the need for monitoring of these neonates after birth.
Early gestational age at delivery, lower birthweight, neonatal morbidity and perinatal deaths occurred more commonly in pregnancies with FGR stages II & III compared to stage I FGR and SGA fetuses (Flowchart 3).

Flowchart 3: Flowchart of management and surveillance in SGA and FGR fetuses
Strengths of the Study
It is a prospective study, there is strict implementation of the inclusion and exclusion criteria.
Advantages of the Study
-
Pregnant women with isolated SGA fetuses were allowed to continue pregnancy till 40 weeks of gestation.
-
It is comfortable to differentiate between SGA and FGR fetuses based on the stage-based classification.
-
Incorporation of stage-based classification for FGR provides uniformity in multiple operator center like our institute.
-
Interventions like Induction of labor were reduced.
-
These outcomes would help in prenatal counseling of couples with FGR fetuses.
Limitations of Study
-
This is institutional data, where tertiary care is offered, so this data cannot be extrapolated to the community.
-
It is an observational study and there is no comparison group with the small study population.
-
To extrapolate the Barcelona protocol in Indian population in clinical practice in the Indian setup, further comparative studies and meta-analysis from various centers are needed.
What is already Known about this Topic
There are various consensus to define, diagnose and classify FGR and there are various studies comparing the perinatal outcomes in SGA, FGR fetuses.
What this Study Adds
Stage-based protocol is a very important attempt to standardize the diagnosis and surveillance of SGA and various stages of FGR, but there is lack of studies to validate these criteria.
Our study is a single-center study that used it for the diagnosis of SGA and stages of FGR.
We have observed that the criteria established for diagnosis and surveillance of SGA and Stages of FGR seem good enough for deciding the surveillance interval.
CONCLUSION
Overall, our findings corroborate the observation that incorporation of Barcelona protocol as a structured antenatal surveillance protocol discriminates between SGA fetuses and Stages of FGR with a higher risk for adverse perinatal outcomes in FGR II & III compared to FGR stage I and SGA fetuses. Prenatal recognition of FGR allows for close monitoring in terms of follow-up with multivessel Doppler as per the stage-based protocol, maternal evaluation of hypertension, and timely delivery. Additional methods of surveillance like AFI, non-stress test (NST), intrapartum monitoring, and NICU care cannot be ignored in spite of using stage based approach. Despite the antenatal recognition of maternal risk factors and diagnosis of FGR with the initiation of increased surveillance, not all perinatal deaths in our study were prevented.
ACKNOWLEDGMENTS
The authors thank the Director of Fetal Medicine, Fetal Medicine Consultants, Staff of Department of Fetal Medicine, Obstetricians, Neonatologists, Fernandez Hospital, Hyderabad, and the women who were enrolled in the study for their support and cooperation.
ORCID
Mounika Kachakayala https://orcid.org/0000-0002-4316-8810
REFERENCES
1. Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 2014;36(2):86–98. DOI: 10.1159/000357592
2. The Investigation and Management of the Small-for-Gestational Age Fetus. London (UK): Royal College of Obstetricians and. Gynecologists(RCOG); 2013. 2nd edn.
3. Lausman A, Kingdom J, Maternal Fetal Medicine Committee. Intrauterine growth restriction: screening, diagnosis, and management. J Obstet Gynaecol Can 2013;35(8):741–748. DOI: 10.1016/S1701-2163(15)30865-3
4. [Intra-uterine growth retardation: guidelines for clinical practice–Short text]. J Gynecol Obstet Biol Reprod (Paris) 2013;42(8):1018–1025. DOI: 10.1016/j.jgyn.2013.09.023
5. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol 2013;121(5):1122–1133. DOI: 10.1097/01.AOG.0000429658.85846.f9
6. Unterscheider J, O’Donoghue K, Daly S, et al. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth 2014;14:63. DOI: 10.1186/1471-2393-14-63
7. Baschat AA, Viscardi RM, Hussey-Gardner B, et al. Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet Gynecol 2009;33(1):44–50. DOI: 10.1002/uog.6286
8. India Birth Rate 1950–2021[Internet]. Available from: https://www.macrotrends.net/countries/IND/india/birth-rate’>India Birth Rate 1950–2019. www.macrotrends.net.
9. Registrar General of India. Sample registration system (SRS) statistical report 2013. New Delhi: 2013.
10. Devi KS, Aziz N, Gala A, et al. Incidence of stillbirths and risk factors at a tertiary perinatal center in Southern India: retrospective observational study. Int J Gynecol and Reprod Sci 2018;1(1):14–22.
11. Singh A, Ambujam K. Maternal socio-demographic determinants and fetal outcome of intrauterine growth restriction. Int J Reprod Contracept Obstet Gynecol. 2018;7:3843–3847. DOI: 10.18203/2320-1770.ijrcog20183805
12. Chauhan SP, Beydoun H, Chang E, et al. Prenatal detection of fetal growth restriction in newborns classified as small for gestational age: correlates and risk of neonatal morbidity. Am J Perinatol 2014;31(3):187–194. DOI: 10.1055/s-0033-1343771
13. Clifford S, Giddings S, Southam M, et al. The Growth Assessment Protocol: a national programme to improve patient safety in maternity care. MIDIRS Midwifery Digest 2013;23(4):516–523.
14. Trudell AS, Cahill AG, Tuuli MG, et al. Risk of stillbirth after 37 weeks in pregnancies complicated by small-for-gestational-age fetuses. Am J Obstet Gynecol 2013;208(5):376.e1–376.e7. DOI: 10.1016/j.ajog.2013.02.030
15. Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013;41(2):233–239. DOI: 10.1002/uog.12371
16. Del Río M, Martínez JM, Figueras F, et al. Doppler assessment of fetal aortic isthmus blood flow in two different sonographic planes during the second half of gestation. Ultrasound Obstet Gynecol 2005;26(2):170–174. DOI: 10.1002/uog.1955
17. Salomon LJ, Alfirevic Z, Berghella V, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2011;37(1):116–126. DOI: 10.1002/uog.8831
18. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 348, November 2006: Umbilical cord blood gas and acid-base analysis. Obstet Gynecol 2006;108(5):1319–1322. DOI: 10.1097/00006250-200611000-00058
19. Şahin Uysal N, Gülümser Ç, Bilgin Yanık F. Maternal and perinatal characteristics of small-for-gestational-age newborns: ten-year experience of a single center. J Turk Ger Gynecol Assoc 2017;18(2):90–95. DOI: 10.4274/jtgga.2016.0228
20. McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol 2009;23(6):779–793. DOI: 10.1016/j.bpobgyn.2009.06.003
21. Thompson JM, Clark PM, Robinson E, et al. Risk factors for small-for-gestational-age babies: The Auckland Birthweight Collaborative Study. J Paediatr Child Health 2001;37(4):369–375. DOI: 10.1046/j.1440-1754.2001.00684.x
22. Teixeira MP, Queiroga TP, Mesquita MD. Frequency and risk factors for the birth of small-for-gestational-age newborns in a public maternity hospital. Einstein (Sao Paulo) 2016;14(3):317–323. DOI: 10.1590/s1679-45082016ao3684
23. Satyavrathan V, Ahmed N, Sundrappa S. Study of perinatal outcomes of pregnancies with intrauterine growth restriction in a tertiary care centre in North Kerala. J Evid Based Med Healthc 2017;4(37):2203–2208.
24. Sharma DD, Chandnani KC. Clinical study of IUGR cases and correlation of Doppler parameters with perinatal outcome. Int J Reprod Contracept Obstet Gynecol 2016;5(12):4290–4296. DOI: 10.18203/2320-1770.ijrcog20164330
25. Shenoy HT, James SX, Shenoy ST. Maternal risk factors and perinatal outcomes in fetal growth restriction using obstetric Doppler in South Kerala, India. Int J Reprod Contracept Obstet Gynecol 2019;8(1):6–13. DOI: 10.18203/2320-1770.ijrcog20185062
26. Gagnon R, Harding R, Brace RA. Amniotic fluid and fetal urinary responses to severe placental insufficiency in sheep. Am J Obstet Gynecol 2002;186(5):1076–1084. DOI: 10.1067/mob.2002.122291
27. Fratelli N, Valcamonico A, Prefumo F, et al. Effects of antenatal recognition and follow-up on perinatal outcomes in small-for-gestational age infants delivered after 36 weeks. Acta Obstet Gynecol Scand 2013;92(2):223–229. DOI: 10.1111/aogs.12020
28. O’Dwyer V, Burke G, Unterscheider J, et al. Defining the residual risk of adverse perinatal outcome in growth-restricted fetuses with normal umbilical artery blood flow. Am J Obstet Gynecol 2014;211(4):420.e1–420.e5. DOI: 10.1016/j.ajog.2014.07.033
29. Seal A, Dasgupta A, Sengupta M, et al. Analysis of fetal growth restriction in pregnancy in subjects attending in an obstetric clinic of a tertiary care teaching hospital. Int J Reprod Contracept Obstet Gynecol. 2018;7:973–80. DOI: 10.18203/2320-1770.ijrcog20180876
30. Sinha S, Kurude VN. Study of obstetric outcome in pregnancies with intrauterine growth retardation. Int J Reprod Contracept Obstet Gynecol. 2018;7:1858–63. DOI: 10.18203/2320-1770.ijrcog20181918
________________________
© The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.