ORIGINAL ARTICLE |
https://doi.org/10.5005/jp-journals-10016-1217 |
Significance of Evaluating Immature Germ Cells during Semen Analysis
Department of Obstetrics and Gynaecology, Faculty of Medicine Ragama, University of Kelaniya, Sri Lanka
Corresponding Author: Anura Dissanayake, Department of Obstetrics and Gynaecology, Faculty of Medicine Ragama, University of Kelaniya, Sri Lanka, Phone: +94 714119634, e-mail: anuradissa@kln.ac.lk
ABSTRACT
Aim: The clinical significance of evaluating non-sperm cells, which include spermatogenic and non-spermatogenic cells, commonly known as “round cells” is not well established. This study aimed to assess the clinical significance of distinct identification of immature germ cells (IGCs) in semen, and their relationship with semen parameters and chromosome maturity.
Materials and methods: A prospective laboratory-based study was carried out using 140 semen samples collected from males attending the subfertility clinic. Semen analysis (SA), evaluation of immature cells, and leukocytes were performed according to standard protocols. The percentage of IGCs calculated by the ratio of IGCs to sperm was used in evaluating above relationships.
Results: Immature germ cells were detected in all semen samples with the mean (SD) 26.09 (23.80)%. Significant increase in IGCs was evident in pathozoospermic group (assessed by triple defects; sperm count, motility, and morphology) compared to normozoospermics; mean (SD), 37.75 (29.95)% vs 18.57 (14.95)%, p < 0.01. Analysis of correlations showed that the decrease in concentration, progressive motility, and normal morphology of spermatozoa were associated with increase in percentage of IGCs, and increased IGCs were accompanied with the significant increase in sperm head immaturity. Differentiation between normozoospermia and pathozoospermia was fairly probable or acceptable at the cut-off value of 15.5% of IGCs using ROC curve, with 0.659 sensitivity and 0.281 specificity.
Conclusion: A high count of IGCs in semen is a good indicator for detecting disturbed testicular niche compartments, and those changes may manifest as pathozoospermia.
Clinical significance: Identifying the different etiologies for this specific situation would help in the proper management of those patients.
How to cite this article: Dissanayake A. Significance of Evaluating Immature Germ Cells during Semen Analysis. Int J Infertil Fetal Med 2022;13(2):42-46.
Source of support: Nil
Conflict of interest: None
Keywords: Immature germ cells, Leukocytes, Semen analysis, Sperm chromatin immaturity
INTRODUCTION
Semen analysis is the first-line investigation in evaluating male fertility potential. The test reveals not only the quality of semen but also the pathophysiological status of the male reproductive system. Accurate SA result together with the patient’s history and physical examination would direct either further investigations or treatment options. Different parameters such as semen volume, pH, liquefaction time, viscosity, sperm count, motility, viability, morphology, presence of agglutination, and non-sperm cells are assessed during routine SA.1 Among them sperm count, motility, and morphology are reported as the most weighty predictors of fertility over others.2-4 However, the norms defined by WHO are only gross references possibly helpful in identifying men whose chances of achieving natural pregnancy are very low or impossible in the case of azoospermia.5 The optional tests evaluating different cellular features or functions are currently used as research tools, and not a part of a routine diagnostic procedure.6 Accurate analysis of basic semen parameters still stands as a cost-effective tool, and conventional SA together with specialized tests are selectively used in some clinics to decide treatment options.
In addition to sperm, non-sperm cells enter into semen at different levels of the reproductive system. The types and numbers may vary among men depending on the nature of internal and external stresses affecting the reproductive system. Despite the differences in morphological characteristics, different cell types are lumped together as “round cells” that include IGCs, isolated sperm heads, and white blood cells (granulocytes, macrophages, monocytes, and lymphocytes). Epithelial cells, ciliary tufts, remnants of Sertoli cells, and squamous cells are rarely observed in some samples.7 Excessive presence of round cells has been interpreted as a sign of inflammation or infection in many cases, and a close relationship has been shown among those components.8,9 WHO guideline (2010) highlights the need for assessment of leukocytes when round cells concentration exceeds 1 × 106. However, there is no consensus in literature on this relationship.10 IGCs are present in almost all semen samples except in obstructive azoospermia or in a few cases of no n-obstructive azoospermia. Excessive number of premature germ cells are released in to the lumen due to Sertoli cell defects or loss of cytoplasmic connections which maintain the germinal epithelium in order.11 In a pilot study, we observed a wide variation of IGCs in different semen samples, and the count is significantly higher in subnormal samples. Hence, we assume that accurate assessment of IGCs may have additional power on predicting semen quality and future fertility potential. In addition, the results may facilitate referring for further diagnoses or treatment options other than use in semen processing at present. To achieve those objectives, we observed the prevalence and types of IGCs in semen of a subfertile male population, and their relationship with semen parameters and sperm chromatin maturity.
MATERIALS AND METHODS
The study consisted of 140 semen samples collected from males attending the subfertility clinic. All procedures were followed in accordance with the ethical standards of the institutional ethics committee. Informed consent was obtained from each participant, and samples were collected into sterile and wide-mouthed containers, after three days of ejaculatory abstinence. Semen parameters were assessed according to the WHO guidelines (5th edition). Immature germ cells and other cell types were assessed in diff-quick stained slides. Immature germ cells were categorized morphologically (size and shape of the cell and nucleus) into two groups, namely; spermatocytes and spermatids. Peroxidase positive granulocytes, identified using peroxidase ortho-toluidine blue technique as described in the WHO manual, were excluded. The IGC count was given as several cells per 100 sperm and presented as a percentage. At least 200 sperm were counted for the accuracy of readings.
The state of sperm chromatin maturity was assessed by the aniline blue staining method. Briefly, semen smeared slides were air-dried and fixed in 3% buffered glutaraldehyde for 30 minutes at room temperature. Slides were stained with 5% w/v acidic aniline blue (pH 3.5) for 5 minutes, and a counter stain was done with 1% eosin for 3 minutes. Two hundred sperms were counted under oil emersion (100×) for differentiating dark blue or immature sperm (lysine rich histones) from pale red-pink or mature sperm (arginine and cysteine rich protamine).
Statistical analysis was performed using the SPSS, version 16.0 computer software (Mariakerke, Belgium). The significance of differences between groups was assessed using independent samples t-test. Correlations were calculated using Pearson’s coefficient test, and receiver operating characteristics (ROC) curves were used to determine the discriminative power between the groups and to identify criterion values. Data are represented as mean (SD), and a p value of <0.05 was considered as statistically significant.
RESULTS
Semen samples had a mean (SD) concentration of 36.72 (2.87) × 10 /mL, motility of 49.33 (2.05)%, and normal morphology of 11.02 (6.03)%. The major group of round cells in semen was IGCs, and were detected in all semen samples (including two azoospermic samples with normal volume). Morphologically these cells could simply be categorized into two groups; spermatocytes and spermatids. Presence of different cell types in semen was expressed as a proportion of cells to total sperm. Percentage of mean spermatocytes (primary and secondary) in semen samples was 9.93 (6.27)% and spermatids was 16.16 (13.49)%. The mean (SD) of total IGCs (both spermatocytes and spermatids) was 26.09 (23.80)%. Peroxidase-positive leukocytes were observed in 51% of samples. In other samples, leukocytes were not detected (not detected in 20 consecutive high power microscopic fields). The mean percentage of leukocytes was 3.40 (3.02)%, and aniline blue positive cells or chromatin immature sperm was 22.12 (1.19)%. Figure 1 shows the different types of non-sperm cells, and immature sperm detected in the study group.
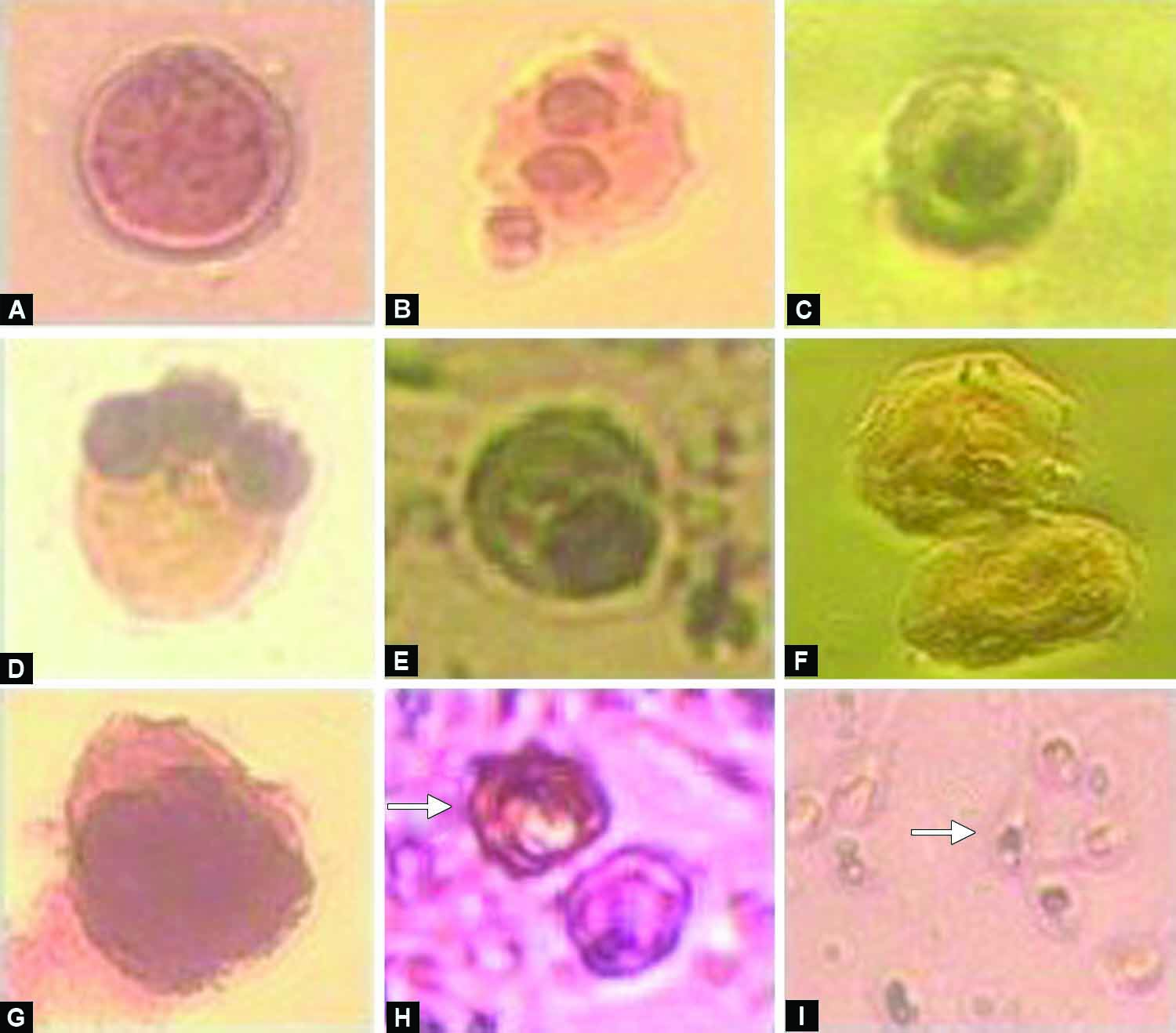
Figs 1A to I: Different types of cells observed in semen samples 200×; (A) Spermatocyte; (B) Dividing spermatocyte; (C) Spermatid; (D) Multinucleated spermatid; (E) Elongating spermatid; (F) Epithelial cells; (G) Macrophage; (H) Granulocytes stained with peroxidase-o-toluidine blue stain (arrow); (I) Sperm stained with aniline blue (arrow indicates chromatin immature sperm)
Group statistics showed that increased rates of spermatocytes, spermatids and total IGCs (collectively spermatocytes and spermatids) are present in pathozoospermic group compared to normozoospermics; mean (SD), 14.97 (12.86)% vs 6.57 (5.12)%, p < 0.01, 22.78 (16.87)% vs 12.00 (8.60)%, p < 0.01 and 37.75 (29.95)% vs 18.57 (14.95)%, p < 0.01, respectively. All groups of IGCs as well as immature sperm were significantly high in oligozoospermic samples compared to samples with normal counts. A similar trend was observed between morphology groups and IGCs, whereas teratozoospermia was accompanied with high counts of spermatids and total IGCs. However, this relationship was not observed with spermatocytes (Table 1).
| Spermatocytes (%) | Spermatids (%) | Total immature germ cells (%) | Immature sperm (%) | |
|---|---|---|---|---|
| Normozoospermic | 6.57 (5.12) | 12.00 (8.60) | 18.57 (14.95) | 19.96 (11.11) |
| Pathozoospermic | 14.97 (12.86)* | 22.78 (16.87)* | 37.75 (29.95)* | 24.87 (12.47) |
| Normal count (mn/mL) | 5.82 (5.18) | 11.90 (8.22) | 17.72 (11.74) | 18.93 (8.51) |
| Oligozoospermic | 19.31 (18.27)* | 25.87 (17.03)* | 45.18 (32.24)* | 28.51 (15.03)* |
| Normal motility (%) | 8.05 (7.14) | 15.03 (12.18) | 23.08 (18.85) | 21.33 (11.86) |
| Asthenozoospermia | 15.96 (13.36) | 19.76 (16.81) | 35.72 (34.03) | 24.28 (12.06) |
| Normal morphology (%) | 8.30 (7.91) | 14.61 (12.82) | 22.91 (20.67) | 20.48 (10.09) |
| Teratozoospermia | 19.73 (18.75) | 25.46 (14.11)± | 45.20 (32.18)± | 30.66 (16.77)± |
*Significant at p = 0.01 level, ±Significant at p = 0.05 level, mn/mL–million per milliliter
There was a strong positive correlation between IGCs and the percentage of poorly condensed sperm or immature sperm assessed by acidic aniline blue stain. On the other hand, we observed a strong negative correlation between IGCs and sperm concentration, and a week negative correlation between immature cells and sperm motility, and morphology (Table 2). The sperm head chromatin immaturity was also associated with decreased sperm count and morphology.
| Spermatocytes (%) | Spermatids (%) | Total immature cells (%) | |
|---|---|---|---|
| Concentration | −0.376* | −0.410* | −0.442± |
| Motility | −0.229± | −0.129 | −0.201± |
| Morphology | −0.381± | −0.458± | −0.472± |
| Leukocytes | 0.151 | −0.100 | 0.008 |
| Sperm immaturity | 0.560* | 0.454* | 0.574* |
*Significant at p = 0.01 level, ±Significant at p = 0.05 level
The discriminating values between different seminal groups were assessed using the ROC curve. We observed that the cut-off value of 15.5% of IGCs was able to fairly discriminate between normozoospermic and pathozoospermc samples with 0.659 sensitivity and 0.281 specificity. The area under the curve was 0.742 with SE ± 0.44 and 95% CI, 0.655–0.828 (Fig. 2). Similarly, it was observed that semen samples fall under the oligozoospermic category when the percentage of IGCs exceeds. The best cut-off for this situation is 28.5% of IGCs with 0.688 sensitivity and 0.151 specificity, and the area under the curve was 0.795 with SE ± 0.52 and CI, 0.693–0.896.
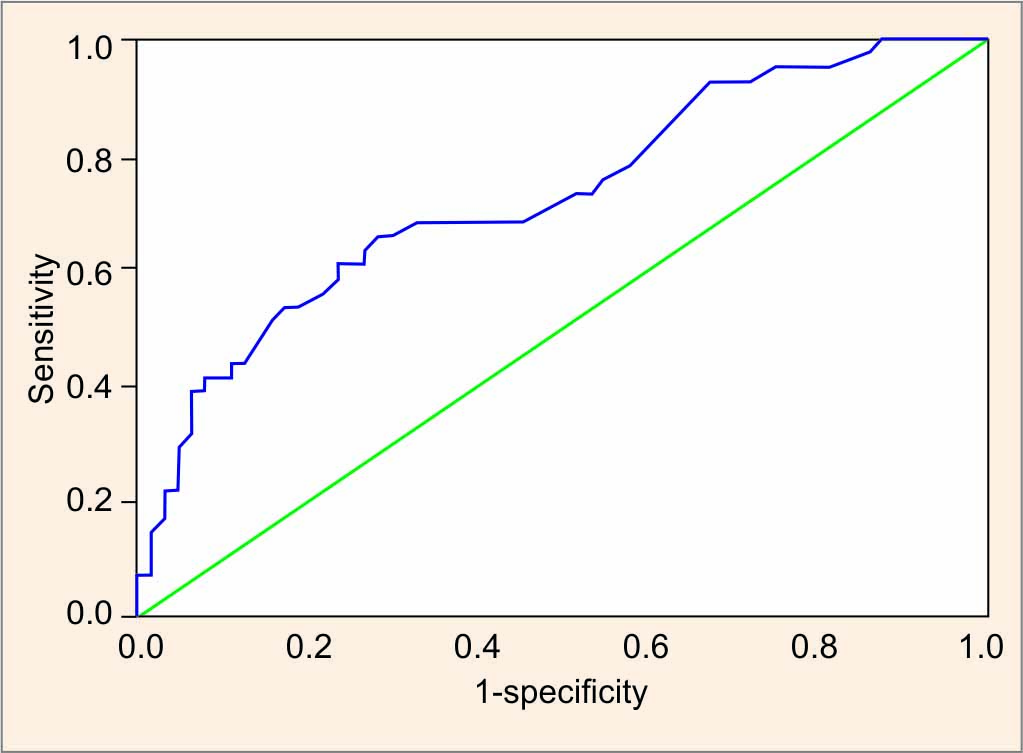
Fig. 2: The receiver operating characteristic (ROC) curve analysis for discriminating between normozoospermia and pathozoospermia by immature germ cells (AUC, area under the curve)
DISCUSSION
Male factor infertility is significantly contributed to the couple’s infertility and improving the quality of subnormal semen to achieve natural pregnancy is a major challenge. This is because lack of well-defined techniques to assess the quality of semen with regard to the true fertility status of men. Conventional SA has low discriminating power between fertile and infertile men and, the validity of other special tests available in evaluating the structure and function of subcellular organelles is controversial. On the other hand, the development of assisted reproductive technologies (ART) that can use fewer or single sperm to achieve pregnancies has decelerated the improvement of diagnostic facilities for men. However, the use of genetically or epigenetically deformed germ cells for the treatment of subfertility using invasive procedures is an open debate in clinical and ethical aspects.11 Thus, improving the diagnostic facilities and treatment options for achieving natural fertilization still have a place considering the cost of ART and the wellbeing of future generations. In conventional SA, the predictive accuracy of natural pregnancy is closely linked with severe oligo, astheno, and teratozoospermia. Other parameters, i.e., volume, pH, round cells, or WBCs may help to increase the predictive power. Assessment of DNA quality is recently recognized as an important measure of fertilizing efficiency of sperm.12
The relationship between IGCs and other semen parameters has gained only a little attention so far. A high count of IGCs in semen would represent different pathophysiological conditions such as Sertoli cell defects, varicocele, endocrine disorders, chromosome abnormalities or gene mutations, and germline cell toxins such as heavy metals, etc.13 It has been suggested that a high number of IGCs in semen may be a sign of the early stage of deteriorating semen quality, and if untreated may lead to azoospermia. On the other hand, IGCs in azoospermic men could be used as a dependable marker for discriminating obstructive and non-obstructive azoospermia.14 This is very useful as a confirmative test avoiding testicular biopsy in many cases. In the present study, our main objective was to examine the clinical significance or utility of detailing IGCs in SA.
The study samples contained IGCs in the range of 2–139% with the mean of 26.09%. These values are considerably high compared to available data, and the count of non-sperm cells is <15% in other similar studies.15,16 Presence of high proportion of pathozoospermc samples (60%) with 15% of severe oligozoospermia is one possible explanation for the high count of IGCs in our study. Round cells were observed only in 5.4% of semen samples by Palermo et al., in contrast, almost all samples contained at least very few numbers of round cells in our population.17 Referring to our results, IGCs in semen has an acceptable or fair discriminating power between normal sperm counts and oligozoospermia, and between normozoospermia and pathozoospermia (detected by the triple defects; low count, motility and morphology).
WHO guidelines recommend converting the values into concentrations when assessing seminal round cells. According to our observations, proportion of IGCs to sperm is the better way of expression compared to calculated concentrations, especially observing relationships with other parameters. This is because concentration is calculated by multiplying the proportion of round cells from sperm concentration. Thus, the calculated concentration is very low in oligozoospermic samples compared to samples with normal sperm count even the round cell number is equal in both samples. Our results indicate a possible insult to the germ cell niche when the proportion of IGCs exceeds 15%. This could be a warning signal of the early stage of deteriorating semen quality even the other semen parameters are unaltered. Supportive pieces of evidence are provided by correlation analyzes, where relationships are negative between IGCs and sperm count, motility, and morphology. Similar observations have been reported by Caşkurlu et al., and the decreasing sperm counts with increasing value of non-spermatozoal cells were also reported by Fedder et al.10,15 Consideration of types of germ cells may have an additional value as we observed a significant negative correlation between spermatocytes count and motility. The possibility of severe disturbances of spermatogenesis in men with mixed populations of both elevated spermatocytes and mature spermatozoa was suggested by Johanisson et al.7 It is anticipated that aneuploidy rate may be high in those sperm. In case of transient injury to germinal epithelium such as flu was proposed to increase round cells, and followed by an increase in sperm production once the cause disappeared.17 Hence, repeated evaluation of round cells or IGCs would provide more information on the state of germinal epithelium, and inclusion of IGCs count instead of gross round cells count in SA would be more useful in the diagnosis of male infertility.
Immature germ cells together with granulocytes may be a sign of infection or inflammation, and chemotactically attracted macrophages with phagocytosed sperm are rarely seen in some samples.8,18 Prevalence of male genital tract infection could vary widely in different populations.19 According to some reports, the majority of leukocytospermic samples are microbiologically negative.18 However, the WHO manual recommends assessing the peroxidase positive granulocytes if round cell count exceeds one million per milliliter. In the present study, we did not observe any relationship between peroxidase-positive leukocytes and IGCs. Leukocytes were detected only in 51% of samples, and in majority of granulocytes positive samples, the round cells count was well below the cut-off value. This is in agreement with observations from several groups, and they have not detected differences of leukocyte count between different seminal groups, or correlations between infections and leukocytes.15,17,20 In contrast, Eggert-Kruse et al. noted a negative correlation between leukocytes and sperm count, and morphology.8
Replacement of histone with protamine is an essential process as it helps to maintain nuclear dimension and head morphology to facilitate sperm motility and genetic stability after fertilization.12 An abnormal chromatin packaging may lead to poor fertility outcomes and may be used as a predictor for the fertilizing capacity of sperm.21,22 Our results of 22% immature heads are closely related to 18% reported by Kim et al., but not with 80% of cells observed by Patil et al.22,23 According to Tomlinson et al. seminal leukocytes had only a little influence on the fertilizing capacity of the spermatozoa, but the large numbers of germline cells were associated with reduced fertilizing capacity in IVF cycles, possibly due to presence of a high count of immature sperm population.24 We observed a strong positive correlation between sperm chromatin immaturity and shedding of IGCs, and negative correlations with sperm count and morphology providing supportive evidence for the above hypothesis. Two other studies have noted a negative correlation between abnormal chromatin structure and morphology.23,25 However, Hammadeh et al. reported that chromatin condensation is completely independent from changes in conventional semen parameters.26
Better discrimination between fertile and sub fertile groups was observed at the cut-off value of 53% of mature heads using ROC curve by Auger et al.27 We could not notice such a difference between normozoospermic and pathozoospermc groups using sperm maturity. Some difficulties in performing aniline blue test such as, difficulty in visualizing and differential counting would be the reasons behind wide variations of results.25
CONCLUSION
The results indicate that elevated IGCs would be a good indicator for detecting disturbed testicular microenvironment. It is better to go into more detailed diagnoses if the rate of IGCs exceeds 15% of sperm in semen. However, there was no relationship between the presence of IGCs and white blood cells. As the high count of IGCs coincide with sperm chromatin immaturity, taking precautions to exclude immature cells would be useful in ART procedures, especially in intracytoplasmic sperm injection (ICSI). Exact causes of shedding of immature spermatogenic cells, long-term consequences in relation to changes in semen quality and subsequent effects on fertility are avenues for further studies.
ACKNOWLEDGMENTS
I acknowledge the technical staff in the laboratory for their assistance in semen analysis.
REFERENCES
1. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed., Geneva: WHO Press; 2010.
2. Bedaiwy MA, Sharma RK, Alhussaini TK, et al. The use of novel semen quality scores to predict pregnancy in couples with male-factor infertility undergoing intrauterine insemination. J Androl 2003;24(3):353-360. DOI: 10.1002/j.1939-4640.2003.tb02682.x.
3. Jedrzejczak P, Hauke GT, Hauke J, et al. Prediction of spontaneous conception based on semen parameters. Int J Androl 2008;31(5):499-507. DOI: 10.1111/j.1365-2605.2007.00799.x
4. Coetzee K, Kruge TF, Lombard CJ. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update 1998;4(1):73-82. DOI: 10.1093/humupd/4.1.73
5. Fertility assessment and treatment for people with fertility problems. National Collaborating Centre for Women’s and Children’s Health, RCOG Press at the Royal College of Obstetricians and Gynaecologists. London (UK): RCOG press; 2004.
6. Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl 2012;14(1):6-13. DOI: 10.1038/aja.2011.58
7. Johanisson E, Campana A, Luthi R, et al. Evaluation of round cells in semen analysis: a comparative study. Hum Reprod Update 2000;6(4):404-412. DOI: 10.1093/humupd/6.4.404
8. Eggert-Kruse W, Bellmann A, Rohr G, et al. Differentiation of round cells in semen by means of monoclonal antibodies and relationship with male fertility. Fertil Steril 1992;58(5):1046-1055. DOI: 10.1016/s0015-0282(16)55458-2
9. de Bellabarba GA, Villarroel TV, Molina CZ, et al. Non-sperm cells inhuman semen and their relationship with semen parameters. Arch Androl 2000;45(3):131-136. DOI: 10.1080/01485010050193896
10. Caşkurlu T, Tasci AI, Samasti M, et al. Immature germ cells in semen and their correlations with other semen parameters. Int Urol Nephrol 1999;31(3):389-393. DOI: 10.1023/a:1007186405678
11. Sharif K, Lewis SEM. Sperm DNA fragmentation testing: to do or not to do? Middle East Fertil Soc J 2013;18(2):78-83. DOI: 10.1016/j.mefs.2013.02.001
12. Singh K, Jaiswal D. Human male infertility: a complex multifactorial phenotype. Reprod Sci 2011;18(5):418-425. DOI: 10.1177/1933719111398148.13
13. Halder A, Jain M, Kumar P. Primary testicular failure: an overview. J Clin Diagn Res 2015(1):3. DOI: 10.4172/2376-0311.1000e105
14. Roy S, Banerjee A, Pandey HC, et al. Application of seminal germ cell morphology and semen biochemistry in the diagnosis and management of azoospermic subjects. Asian J Androl 2001;3(1):55-62.
15. Fedder J, Askjaer SA, Hjort T. Nonspermatozoal cells in semen: relationship to other semen parameters and fertility status of the couple. Archives of andrology 1993;31(2):95-103. DOI: 10.3109/01485019308988386
16. Patil PS, Humbarwadi RS, Patil AD, et al. Immature germ cells in semen—correlation with total sperm count and sperm motility. J Cytol 2013;30(3):185-189. DOI: 10.4103/0970-9371.117682.17
17. Palermo GD, Neri QV, Cozzubbo T, et al. Shedding light on the nature of seminal round cells. PLoS ONE 2016;11(3):e0151640. DOI: 10.1371/journal.pone.0151640
18. Wolff H. The biologic significance of white blood cells in semen. Fertil Steril 1995;63(6):1143-1157. DOI: 10.1016/s0015-0282(16)57588-8
19. Wang AW, Politch J, Anderson D. Leukocytospermia in male infertility patients in China. Andrologia 1994;26(3):167-172. DOI: 10.1111/j.1439-0272.1994.tb00783.x
20. Bassol S, Recio R, De La Cruz DL. Immature spermatogenic cells and leucocytes in normaland abnormal semen. Arch Androl 1990;25(2):115-120. DOI: 10.3109/01485019008987602.
21. Al-Ebrahimi, Al-Sultani YK. Sperm chromatin maturity assay by aniline blue dye and it’s correlation with the result of intracytoplasmic sperm injection. Med J Babylon 2015;12:174-181.
22. Patil P, Bambulkar S, Ajgaonkar S, et al. DNA fragmentation index (DFI) of human semen by modified aniline blue method. Cibtech J Bio-Prot 2013;2:1-5.
23. Kim HS, Kang MJ, Ah Kim S, et al. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin Exp Reprod Med 2013;40(1):23-28. DOI: 10.5653/cerm.2013.40.1.23
24. Tomlinson MJ, Barratt L, Bolton AE, et al. Round cells and sperm fertilizing capacity: the presence of immature germ cells but not seminal leukocytes are associated with reduced success of in vitro fertilization. Fertil Steril 1992;58(6):1257-1259. DOI: 10.1016/s0015-0282(16)55583-6
25. Sellami A, Chakroun N, Ben Zarrouk S, et al. Assessment of chromatin maturity in human spermatozoa: Useful aniline blue assay for routine diagnosis of male infertility. Adv Urol 2013;2013:578631. DOI: 10.1155/2013/578631
26. Hammadeh ME, Zeginiadov T, Rosenbaum P, et al. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch Androl 2001;46(2):99-104. DOI: 10.1080/01485010117363
27. Auger J, Mesbah M, Huber C, et al. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected in fertile men. Int J Androl 1990;13(6):452-462. DOI: 10.1111/j.1365-2605.1990.tb01052.x
________________________
© The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.